IJMS | Free Full-Text | Modeling pKa of the Brønsted Bases as an Approach to the Gibbs Energy of the Proton in Acetonitrile

Higher-Order Cyclopropenimine Superbases: Direct Neutral Brønsted Base Catalyzed Michael Reactions with α-Aryl Esters | Journal of the American Chemical Society

Higher-Order Cyclopropenimine Superbases: Direct Neutral Brønsted Base Catalyzed Michael Reactions with α-Aryl Esters | Journal of the American Chemical Society

Mono‐Phosphazenyl Phosphines (R2N)3P=N–P(NR2)2 – Strong P‐Bases, P‐Donors, and P‐Nucleophiles for the Construction of Chelates - Kögel - 2020 - Zeitschrift für anorganische und allgemeine Chemie - Wiley Online Library

Phosphazene base-catalyzed hydroamination of aminoalkenes for the construction of isoindoline scaffolds: Application to the total synthesis of aristocularine - ScienceDirect

A New Strategy for Deprotonative Functionalization of Aromatics: Transformations with Excellent Chemoselectivity and Unique Regioselectivities Using t-Bu-P4 Base | Journal of the American Chemical Society

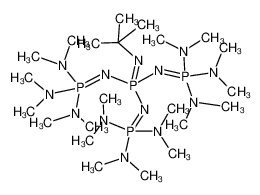
2.12 Binding motives in some of SCHWESINGER's Px-bases. The arrangement... | Download Scientific Diagram

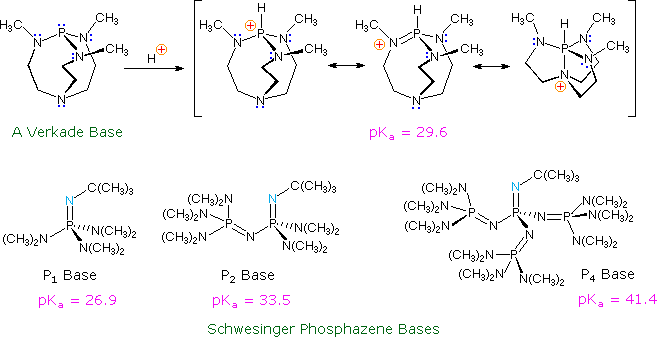
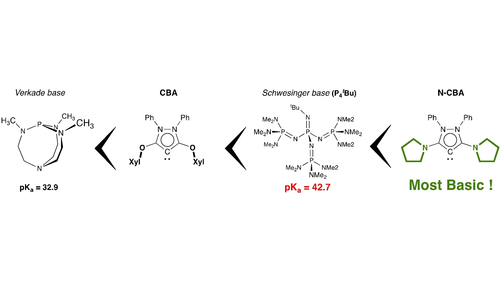
Phosphorus‐Containing Superbases: Recent Progress in the Chemistry of Electron‐Abundant Phosphines and Phosphazenes - Weitkamp - 2021 - Chemistry – A European Journal - Wiley Online Library
![Generation and Applications of the Hydroxide Trihydrate Anion, [OH(OH2)3]−, Stabilized by a Weakly Coordinating Cation - Weitkamp - 2019 - Angewandte Chemie International Edition - Wiley Online Library Generation and Applications of the Hydroxide Trihydrate Anion, [OH(OH2)3]−, Stabilized by a Weakly Coordinating Cation - Weitkamp - 2019 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/b855dc10-9180-4f0d-94a5-fa0c876bdd18/anie201908589-toc-0001-m.jpg)
Generation and Applications of the Hydroxide Trihydrate Anion, [OH(OH2)3]−, Stabilized by a Weakly Coordinating Cation - Weitkamp - 2019 - Angewandte Chemie International Edition - Wiley Online Library

Intramolecular Alkene Hydroamination with Hybrid Catalysts Consisting of a Metal Salt and a Neutral Organic Base - Stegner - 2020 - European Journal of Inorganic Chemistry - Wiley Online Library
Angewandte Chemie on Twitter: "Stable Singlet Carbenes as Organic Superbases (Bertrand) @UCSDChemBiochem @Rodolphe_Jazzar https://t.co/1KmUvGHY5h https://t.co/fiCQLQojAV" / Twitter

A New Synthetic Pathway to the Second and Third Generation of Superbasic Bisphosphazene Proton Sponges: The Run for the Best Chelating Ligand for a Proton | Journal of the American Chemical Society

Phosphorus‐Containing Superbases: Recent Progress in the Chemistry of Electron‐Abundant Phosphines and Phosphazenes - Weitkamp - 2021 - Chemistry – A European Journal - Wiley Online Library