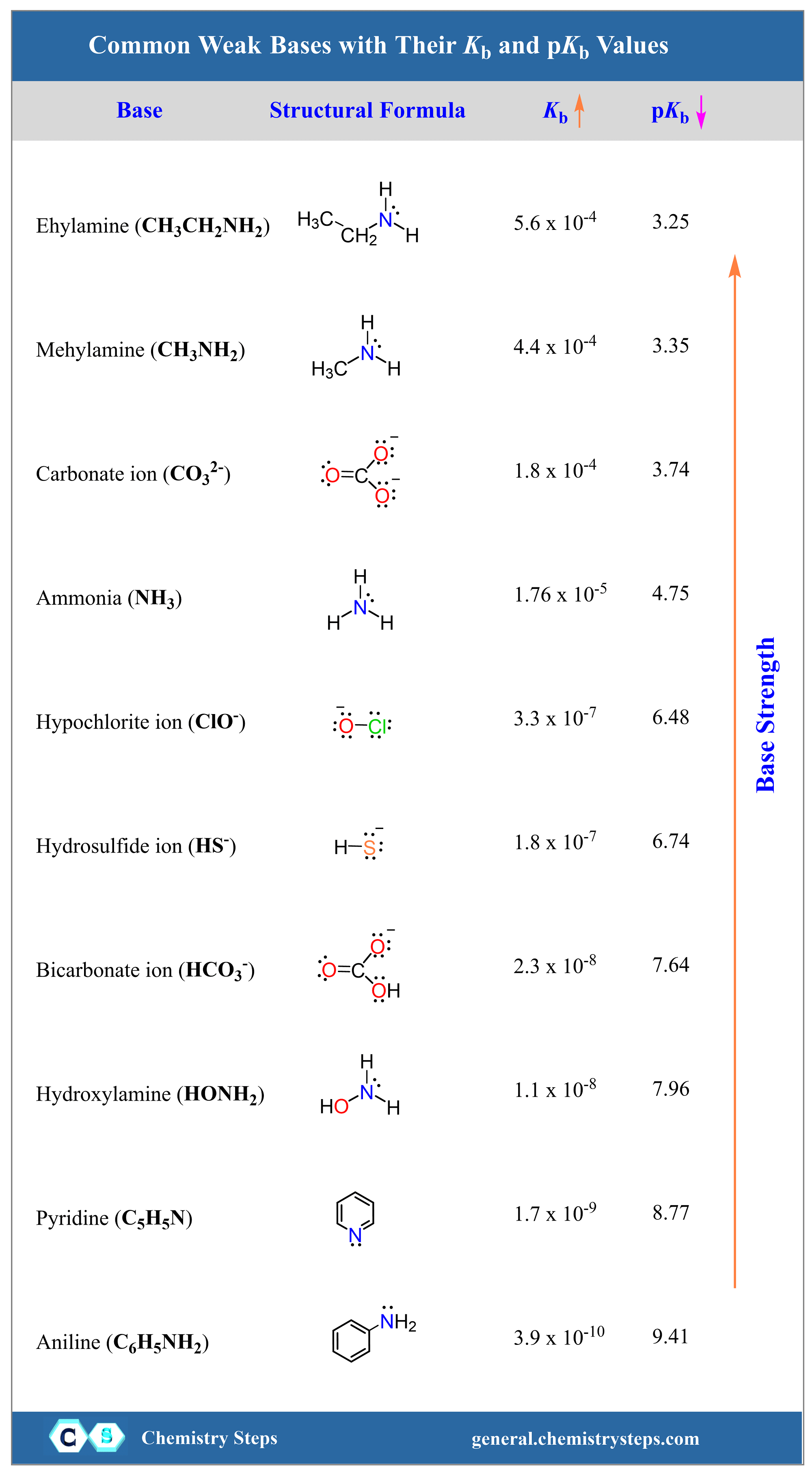
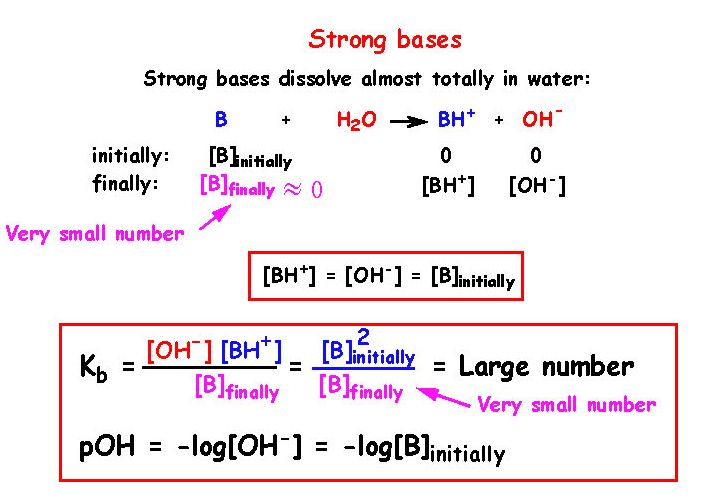
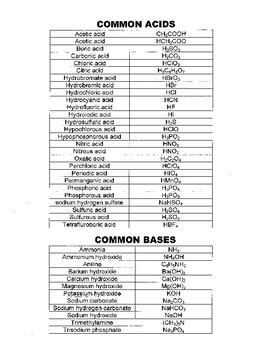
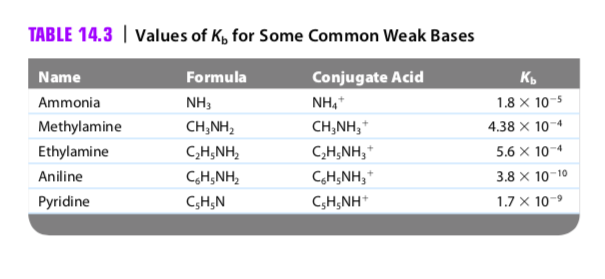
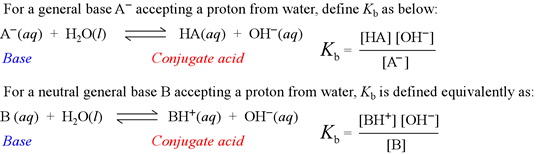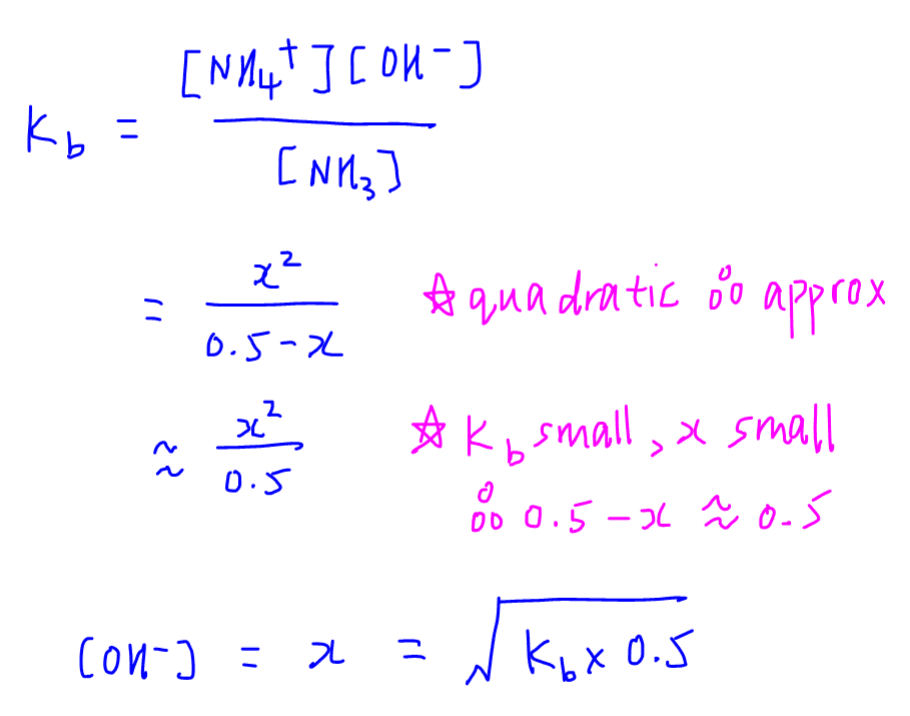![SOLVED: What is the Kb for a base B, if the equilibrium concentrations are [B] = 0.759 M, [HB+] = 0.00254 M, and [OH-] = 0.00254 M? Report your answer in scientific SOLVED: What is the Kb for a base B, if the equilibrium concentrations are [B] = 0.759 M, [HB+] = 0.00254 M, and [OH-] = 0.00254 M? Report your answer in scientific](https://cdn.numerade.com/ask_previews/654acb1d-ee25-40a7-bdff-b3249147ff4c_large.jpg)
SOLVED: What is the Kb for a base B, if the equilibrium concentrations are [B] = 0.759 M, [HB+] = 0.00254 M, and [OH-] = 0.00254 M? Report your answer in scientific
![18.2 Find relative strengths of acids and bases using Ka, Kb, pKa and pKb [IB Chemistry HL] - YouTube 18.2 Find relative strengths of acids and bases using Ka, Kb, pKa and pKb [IB Chemistry HL] - YouTube](https://i.ytimg.com/vi/gUpWOaxW8bE/maxresdefault.jpg)
18.2 Find relative strengths of acids and bases using Ka, Kb, pKa and pKb [IB Chemistry HL] - YouTube