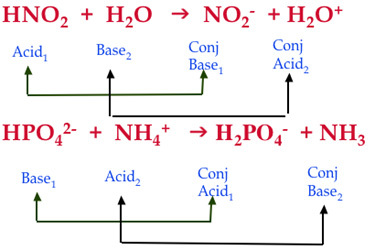
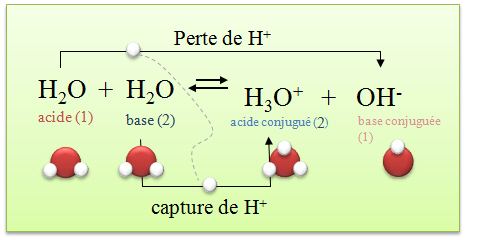
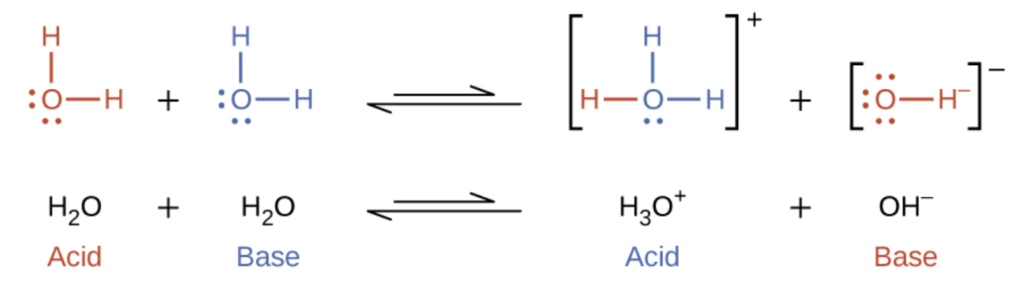
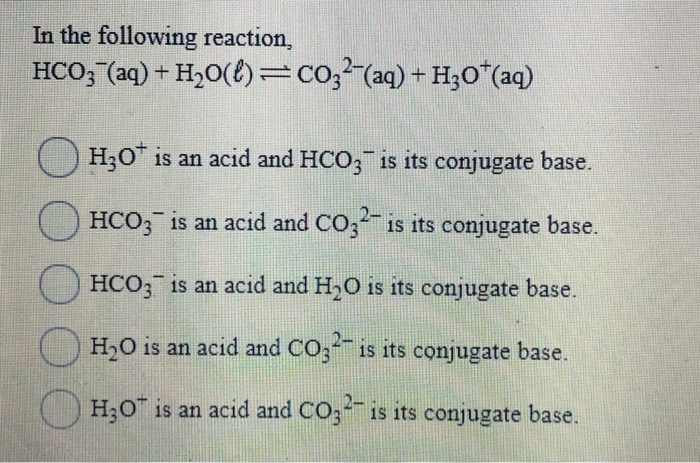G) Sels Dans le chapitre 1, Acide + Base Sel + Eau HCl(aq) + NaOH(aq) NaCl(aq) + H2O Les sels sont des électrolytes forts qui se dissocient entièrement. - ppt video online télécharger
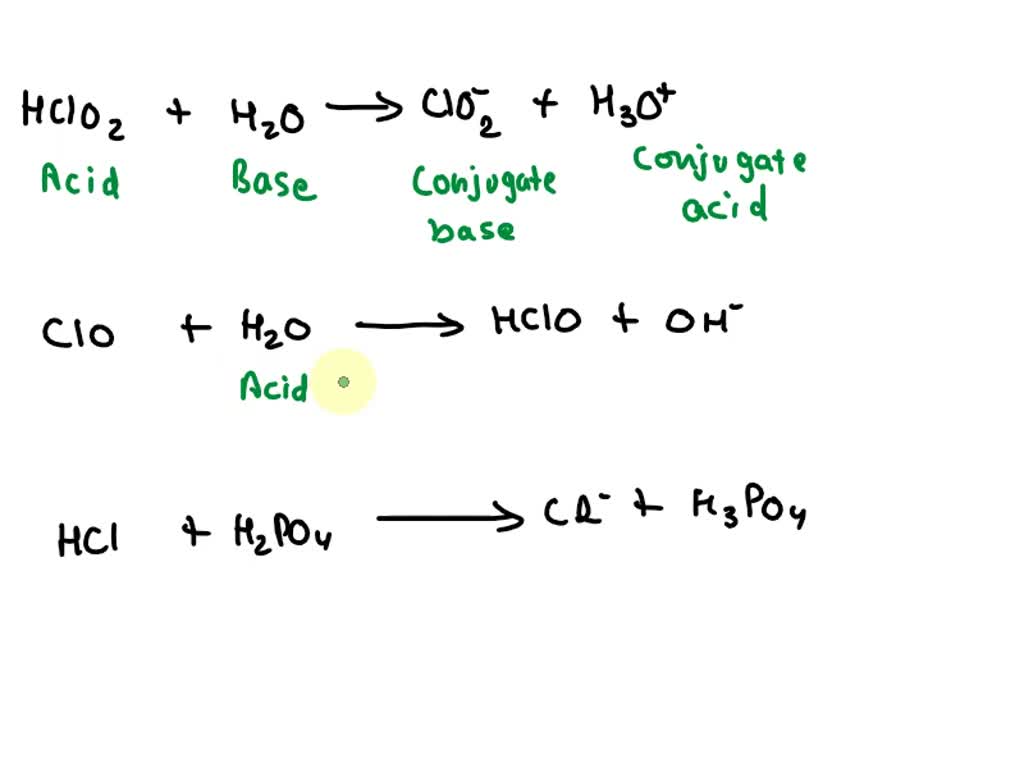
SOLVED: Complete the table Equation Acid Base Conjugate base Conjugate acid HClO2 + H2O → ClO−2 + H3O+ ClO− + H2O → HClO + OH− HCl + H2PO−4 → Cl− + H3PO4